scheme
Abstract
CK2 is a S/T protein kinase, whose aberrant activity is related to a plethora of human diseases, ranging from cancer to virus infections and psychiatric disorders. It is a constitutively active enzyme, with no obvious mechanism of regulation, and, although in many cases its pathologically high activity can be explained by a high CK2 protein level, the reasons of its abnormal up-regulation are largely unknown. It has been reported that CK2 association to specific binding proteins may enhance its activity, but a detailed investigation on this possible mechanism of regulation has never been performed.
Beside up-regulation, mutations of the CK2 gene CSNK2A1 have been recently described in the neurodevelopment disorders Okur-Chung (OCNDS). Only preliminary biochemical characterization of some of these mutants has been reported. We produced some of them, and preliminary results show they have impaired activity.
Within this project, we aim to develop a novel platform based on protein immobilization as well as advanced crystallization and characterization techniques including also the use of synchrotron radiation, to perform structural/functional studies on:
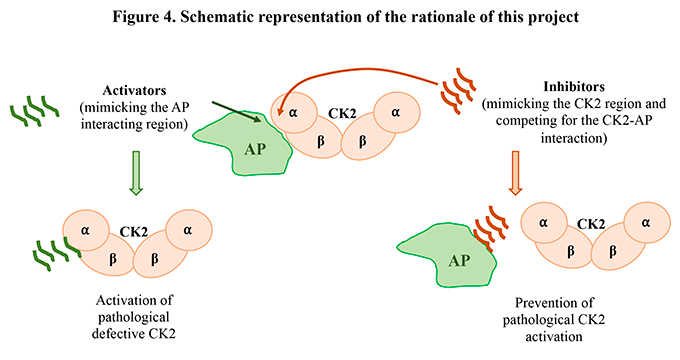
1. CK2 complexes with cellular proteins expected to produce an activator effect. This would allow: (i) to design molecules preventing/disrupting such interactions, thus protecting against aberrant activation of CK2; (ii) by simulating the interacting regions, to obtain small CK2 activator molecules, useful in case of too low CK2 activity;
2. CK2 natural mutants recently found in OCNDS patients, to characterize the biochemical properties and possible conformational changes of relevant cases, with respect to the wild-type protein, and explore hypotheses of future therapeutic interventions.
The three research units (UniPD, UniGE and UniBO) will synergistically contribute according to their specific expertise.
The project is organized into 4 work packages (WPs): production of CK2 (wild-type and natural mutants) and its possible activator partners, biochemical characterization and in cells validation experiments (WP1); stability and morphological studies on selected CK2-activator partners (WP2); structural determination of the most stable CK2-complexes and its natural mutants by X-ray diffraction applying the crystallization through the Langmuir–Blodgett nanotemplate method or by the use of functionalized surfaces, and/or by complementary in solution techniques such as Small Angle X-ray Scattering and Cryo-Electron Microscopy (WP3); management, dissemination and exploitation of the project outcomes (WP4).
The achievements of this project are expected to have a high impact in biomedical field aiming not only at improving the knowledge on a kinase strongly implicated in diseases, but also at identifying new possible therapeutic strategies.
Scientific coordinator for the Department
Prof. Simona Fermani (local coordinator)
Partnership
Alma Mater Studiorum - Università di Bologna (Italy)
Università degli Studi di PADOVA
Università degli Studi di GENOVA
